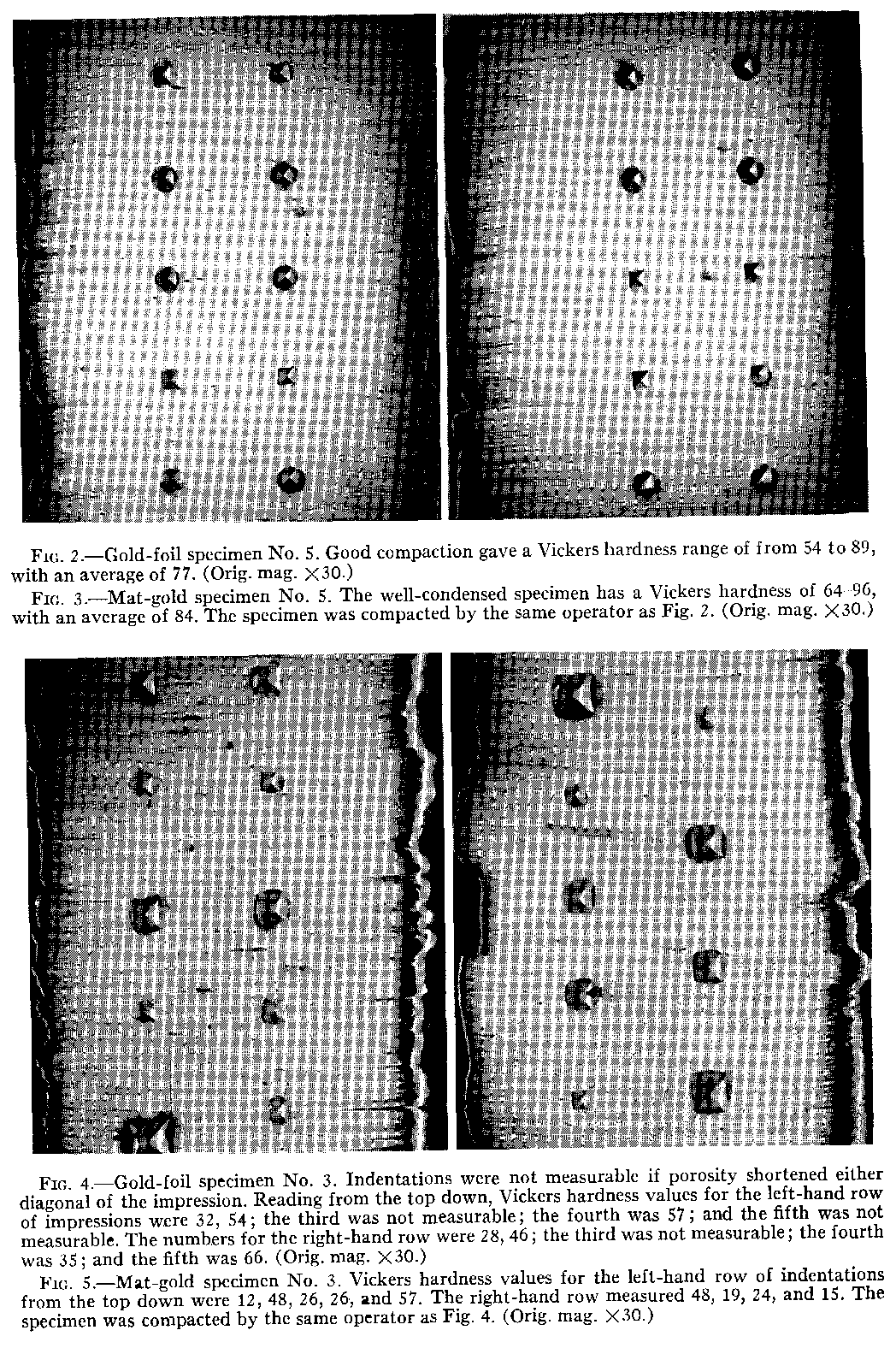
gold contained broken crystal fibers. Porosities were located between the layers of foil, around the mat-gold crystallites, and in the sides of the diamond pyramid hardness impressions.
An unexpected feature was the presence of imprints from the condenser. They were distinguished by the shape of the serrations on the face of the condenser nib. Smooth, dense gold was formed at the base of the imprints under the impact of the condenser. This was the most solid gold in the specimens.
SUMMARY
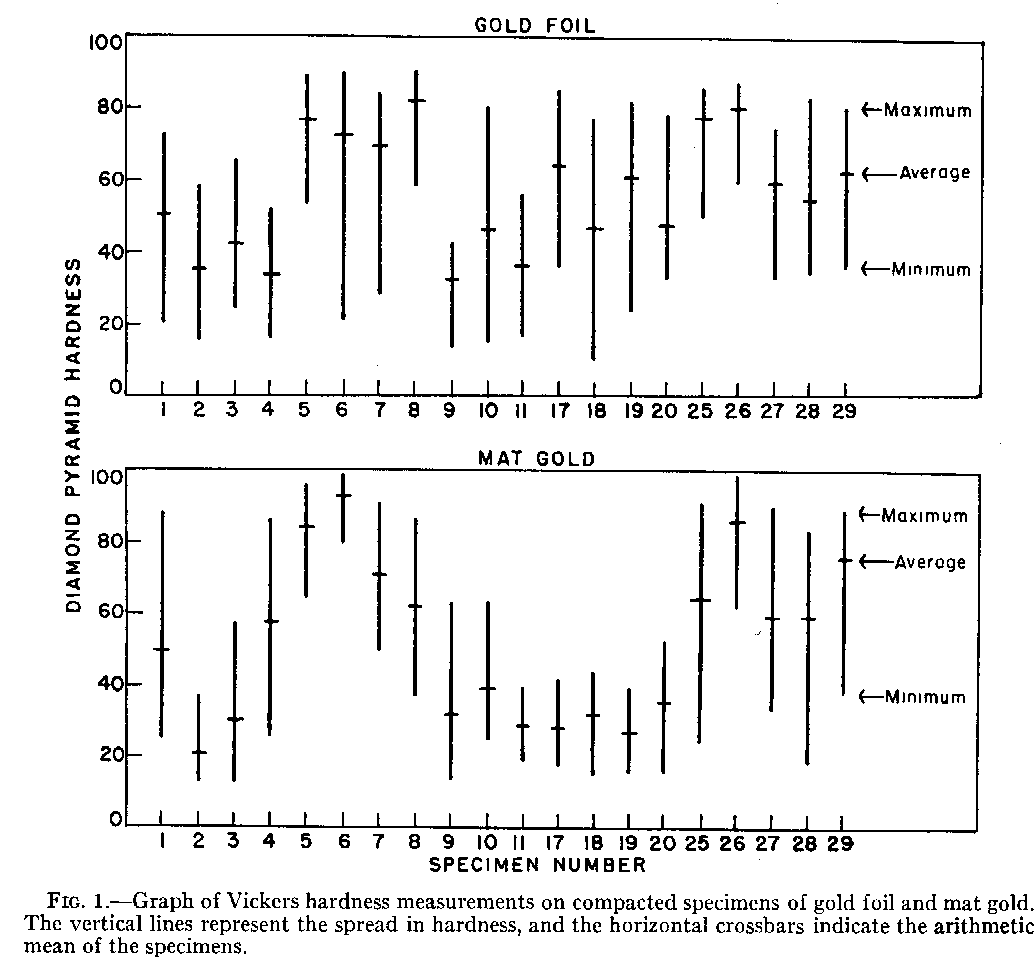
The compaction of gold foil and mat gold into a cavity created similar structures of dense gold and voids. Hardness measurements varied in numerical values because of porosities. Although qualified numbers were difficult to obtain, the hardness indentations were useful for mapping hard and soft areas in specimens. A more convenient method was to remove the surface with aqua regia and examine the underlying structures with the microscope. Results indicated that the theoretical ideal of a dense and solid gold restoration was not achieved with either gold foil or mat gold.
The authors wish to thank Drs. A. F. Dolan, J. M. Grey, L. E. Ostlund, and Mr. Charles Schroeter, of the School of Dentistry, for their help in making the specimens, and Dr. Earl C. Roberts, professor of metallurgical engineering, for his assistance in the project.
REFERENCES
1. STIBBS, G. D. Appraisal of the Gold Foil Restoration, J. Am. Acad. Gold Foil Operators, 2:20, 1959.
2. Hodson, J. T., and STIBBS, G. D. Structural Density of Compacted Gold Foil and Mat Gold, J. D. Res., 39:765, 1960 (abstr.).
3. Hodson, J. '1'. Microstructure of Gold Foil and Mat Gold, D. Progress, 2:55, 1961.
4. KEHL, G. L. Principles of Metallograpltic Laboratory Practice, chap. 1, p. 6. 3d ed. New York: Mc-Graw-Hill Book Co., Inc., 1949.
5. SKINNER, E. W., and PHILLIPS, R. W. The Science of Dental Materials, chap. 19. 5th ed. Philadelphia: W. B. Saunders Co., 1960.
6. PEYTON, F. A., ANTHONY, D. H., ASGAR, K., CHARBENAU, G. T., CRAIG, R. G., and MYERS, G. E. Restorative Dental Materials, chap. 8. St. Louis: C. V. Mosby Co., 1960.
7. SOUDER, W., and PAFFENBARGER, G. C. Physical Properties of Dental Materials, pp. 34-36, 54-56.
(National Bureau of Standards Circ. C433.) Washington, D.C.: Government Printing Office, 1942.
8. HARTMAN, L. L. Controlling Factors in Manipulation of Gold Foil, J.A.D.A., 21.:816, 1934.
9. SMITH, J. C. The Chemistry and Metallurgy of Dental Materials, pp. 45-47. Oxford: Blackwell Scientific
Publications, Ltd., 1946.
10.Rule, R. W. Preliminary Report of Tests Made To Determine Physical Properties and Clinical Values of Gold and Platinum Foil, Foil,J.A.D.A., 23:93, 1936
11. Gold Foil and Platinum-centered Foil: Methods of Condensation, ibid., 24:1783, 1937.
12. A Further Report on Physical Properties and Clinical Values of Platinum-centered Gold Foil as Compared to Pure Gold Filling Materials, ibid., p. 583.
13. SHELL, J. S. Properties and Microscopic Structure of Gold and Gold-Platinum Foils, J.A .D.A., 24; 596, 1937.
14. Physical Properties of Malleted Gold Foil and 24k. Gold Fillings. (Research Department Report.) Hartford: J. M. Ney Co., June 25, 1937.
15. Mat Gold. (Research Report No. 1002.) Buffalo: Williams Gold Refining Co.
16. STROSNIDER, C. W. Comparison of Specific Gravities and Hardness of Cohesive Gold Foil Malleted with Various Types of Condensers, J. D. Res., 24:61, 1945.
17. Koser, J. R., and INGRAHAM, R. Mat Gold Foil with a Veneer Cohesive Gold Foil Surface for Class V Restorations, J.A.D.A., 52:714, 1956.
18. KRAMER, W. S., TRANDELL, T. R., and DIEFENDORF, W. L. A Comparative Study of the Physical Properties of Variously Manipulated Gold Foil Materials, J. Am. Acad. Gold Foil Operators, 3:8, 1960.
19. A.S.T.M. Standards 1958, Part 3: Methods of Testing Metals (except Chemical Analysis), pp. 52-57. Philadelphia: American Society for Testing Materials, 1958.